- Student test on a lemon battery
- Teacher notes and answers for this test
- Buy using PayID 47065111373 OR
- Your files will be emailed to you
- Download the App by pointing your camera at the QR code

Parent and Teacher Notes
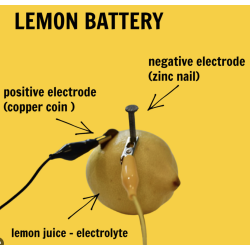
This experiment allows your child to build a simple lemon battery to understand how electricity and batteries work. Just like a battery, a lemon can store electrical energy in chemical form. By combining the lemons with zinc and copper, we can transform the chemical energy into electricity and turn on a light.
Scientific concepts covered:
Battery: A device that stores chemical energy that can be converted into electricity.
Zinc: A metal commonly used in batteries. It allows us to store a lot of energy in a small space inside the battery.
Copper Wire: A metal wire. Copper is great at conducting electricity, so it is often used in batteries and circuits.
Electrical Energy: Electrical energy is the flow of tiny particles called electrons. We use it to power electrical devices, like lights and TVs. In nature, electrical energy can be seen in lightning!
Chemical Energy: Chemical energy is stored in the bonds between molecules. It can be released by chemical reactions.
Electricity: Electricity travels in electric currents. Electric currents are generated when electrons move around.
Production Notes
Image displayed for illustrative purposes only. if you are the copyright owner, please contact us.

Parent and Teacher Notes
This experiment allows your child to build a simple lemon battery to understand how electricity and batteries work. Just like a battery, a lemon can store electrical energy in chemical form. By combining the lemons with zinc and copper, we can transform the chemical energy into electricity and turn on a light.
Scientific concepts covered:
Battery: A device that stores chemical energy that can be converted into electricity.
Zinc: A metal commonly used in batteries. It allows us to store a lot of energy in a small space inside the battery.
Copper Wire: A metal wire. Copper is great at conducting electricity, so it is often used in batteries and circuits.
Electrical Energy: Electrical energy is the flow of tiny particles called electrons. We use it to power electrical devices, like lights and TVs. In nature, electrical energy can be seen in lightning!
Chemical Energy: Chemical energy is stored in the bonds between molecules. It can be released by chemical reactions.
Electricity: Electricity travels in electric currents. Electric currents are generated when electrons move around.
Production Notes
Image displayed for illustrative purposes only. if you are the copyright owner, please contact us.
AR2 - How do you build a lemon battery?
- Brand: Kilbaha Education
- Product Code: How do you build a lemon battery?
- Availability: In Stock
-
$10.00
Related Products
Tags: Augmented Reality (AR)